August 2022 - Newsletter
Included in this newsletter
- Upcoming Changes to the HIPAA Regulations
- Eleven Enforcement Actions Uphold Patients’ Rights Under HIPAA
- DMEPOS Error Rates
- DiversifyRx Pharmacy Profit Summit Recap
- Calling All Compounders!!
- Compliance Portal® Spotlight- Fraud, Waste, & Abuse Prevention
- Updated Compliance Forms
- Updated Policies & Procedures

Upcoming Changes to the HIPAA Regulations
We are expecting the new HIPAA regulations to be released soon. Some key changes are response time for requests to access PHI will change from 30 days to 15 days; defining electronic health record (EHR), reducing the burden of identity verification on the patients; and posting estimated fee schedules for PHI access and disclosures on the facility’s website. The HIPAA program will be updated as soon as the final version becomes available.
In addition, if patients request their prescription files, ask them to complete the Access to Records form in HIPAA, Chapter 1. Do not hesitate to give these files. The Office of Civil Rights (OCR) focus is on patient complaints for access to records.
Eleven Enforcement Actions Uphold Patients’ Rights Under HIPAA
The Office of Civil Rights continues the HIPAA Right of Access Initiative. "It should not take a federal investigation before a HIPAA-covered entity provides patients, or their personal representatives, with access to their medical records," said OCR Director Lisa J. Pino. "Health care organizations should note that there are now 38 enforcement actions in our Right of Access Initiative and understand that OCR is serious about upholding the law and peoples' fundamental right to timely access to their medical records."
The latest eleven enforcement actions are against small providers such as dentists, nursing homes, optometrists, psychiatrists, podiatrists, and surgical centers. As you can see, OCR concentrates on small and mid-size healthcare providers.
The answer is always YES if a patient requests medical or pharmacy records access. You do not have to provide the patient's request on the spot unless you want to. If it will take time to gather the patient’s records, use the Request to Access Patient Records form in the HIPAA Compliance Program, Chapter 1, Item 4b.
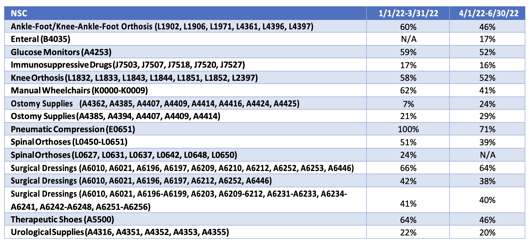
DMEPOS Error Rates
The following chart shows the error rates on certain DME supplies. These errors are almost all documentation errors. Please ensure the correct documentation is prepared and on hand. Refer to the DMEPOS program, Sections 2A - Dispensing and 2A Supplement – Dispensing Documents. The documentation requirements changed on January 1, 2020. Certificates of Medical Necessity (CMN) and DME Information Forms (DIF) will be eliminated as of January 1, 2023.
DiversifyRx Pharmacy Profit Summit Recap
Jenny and Ally attended the Pharmacy Profit Summit, held by the lovely folks at DiversifyRx, in Dallas early this month.
The conference had about 400 independent pharmacy attendees eager to set themselves apart from other pharmacies. It was great talking to people to learn what they are doing to keep their business relevant and profitable. Bobbie Barbrey from Medicap Pharmacy in Raleigh, NC, shared that if he were only filling scripts, he would be out of business by now. He has diversified his pharmacy and found ways to generate revenue that are not only keeping his store afloat, but profitable.
It was great seeing so many of our clients there, and we recommend that if you didn’t attend this year, you look at attending their next conference on August 4-5, 2023.
The Pharmacy Podcast Network covered the event and released a podcast at the event, you can check it out here -> Pharmacy Podcast Network-Pharmacy Profit Summit.
Calling All Compounders!!
Do you compound hormones or do you know physicians who prescribe compounded hormones? The Alliance for Pharmacy Compounding (APC) is asking National Community Pharmacist Association (NCPA) members to send prescribing physicians a sign-on letter for them to send to the Food and Drug Administration Commissioner (FDA), Robert Califf, (with copies going to congressional leaders) urging the commissioners not to restrict compounded hormone therapy based on a discredited report it commissioned and, instead, to base any decision about compounded hormones on solid evidence. APC's letter also calls on FDA to avoid any action that might impede patient access to life-enhancing compounded hormones. For more information and to see the letter, click here. NCPA, August 10, 2022
Compliance Portal® Spotlight- Fraud, Waste, & Abuse Prevention
The Fraud, Waste & Abuse Prevention (FWA) program is one of the most important items that a pharmacy or DMEPOS facility can have and it is also one of the easiest to use. The FWA program is important because it includes:
-
The OIG/SAM Exclusion verification must be completed monthly. Pharmacy Benefit Managers (PBM) and accrediting organizations require proof of this monthly verification. Failure to have these reports on hand (paper or electronic) can cause all reimbursements for Medicare Part B, Part C, and Part D for that period to be returned. This can be devastating to a facility. When the inspection occurs, you either have it or you don’t. This report takes seconds to run and automatically downloads the report with the facility name and date.
-
The FWA policies and procedures are the most requested policies and procedures by all inspecting and auditing organizations.
Updated Compliance Forms
Pharmacy
-
Patient Termination Letter - Medication Non-Compliance (NEW)
-
PatientTerminationLetter– UnpaidInvoices (NEW)
Immunizations
- Monkeypox Standing Order
Updated Policies & Procedures
DMEPOS
-
Patient Intake Durable Medical Equipment
-
Performance Management Plan
Fraud, Waste, & Abuse
-
Fraud Waste and Abuse Prevention Training
HIPAA
-
Complaints to Covered Entity
-
Contingency Plan
-
Safeguards
-
Sanctions
-
Uses and Disclosures to Carry Out Treatment, Payment, or Health Care Operations
Human Relations
-
New Workforce Member Hiring Process
-
Sanctions
-
Workplace Violence
OSHA
-
Bloodborne Pathogens and Hazard Communications
-
Facility Safety Program
-
Fire Prevention
-
Workplace Violence
Pharmacy
-
Performance Management Plan
Enforcement
-
Justice Department Charges Dozens for $1.2 Billion in Health Care DMEPOS Fraud
-
Walgreens fueled San Francisco's opioid crisis with thousands of 'suspicious orders,' judge rules